Lifesaving solution dramatically reduces severe bleeding after childbirth
A trial of a set of interventions to manage postpartum haemorrhage, published in the New England Journal of Medicine, found a 60% reduction in heavy bleeding.
A new solution, known as E-MOTIVE, could provide a major breakthrough in reducing deaths from childbirth-related bleeding, according to a landmark study published today by researchers from the World Health Organisation (WHO) and the University of Birmingham.
Postpartum haemorrhage (PPH) - defined as the loss of more than 500 mL of blood within 24 hours after birth - is the leading cause of maternal mortality worldwide. It affects an estimated 14 million women each year and results in around 70,000 deaths – mostly in low and middle-income countries - equivalent to 1 death every 6 minutes.
The study, which involved over 2,00,000 women in four countries, found that objectively measuring blood loss using a simple, low-cost collection device called a 'drape' and bundling together WHO-recommended treatments - rather than offering them sequentially - resulted in dramatic improvements in outcomes for women. Severe bleeding – when a woman loses more than a litre of blood after birth - was reduced by 60%, and they were less likely to lose their life.
There was also a substantial reduction in the rate of blood transfusions for bleeding, which is of particular importance in low-income countries where blood is a scarce and expensive resource.
Currently, a major challenge in responding to PPH is that it is often detected too late to respond effectively. Most providers use visual inspection to assess bleeding, which tends to underestimate blood loss and can lead to life-threatening delays in treatment. When treatment is provided, this is typically done in a sequential manner with gaps between each intervention – costing more time if the first options are not effective.
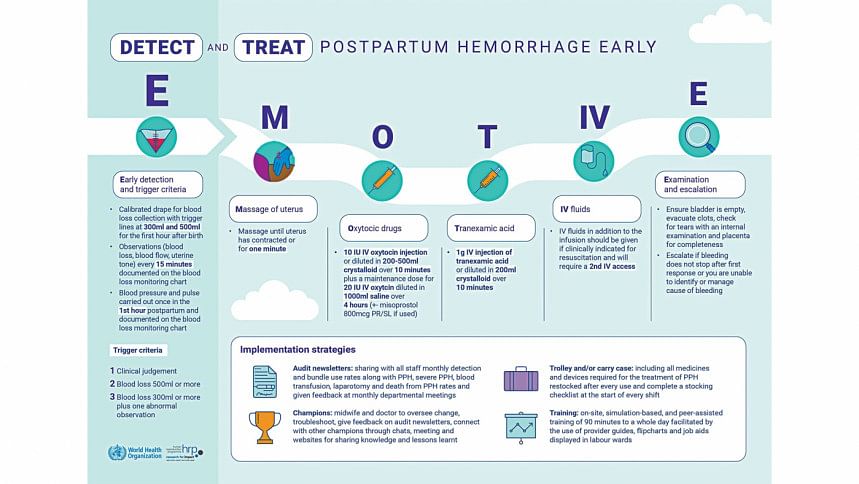
The recommended E-MOTIVE package includes early and accurate detection of PPH using a blood-collection drape. This is complemented by an immediate treatment bundle where indicated, including uterine massage, medicines to contract the womb and stop the bleeding, intravenous fluid administration, an examination and, when needed, escalation to advanced care.
In the trial, the E-MOTIVE intervention was supported with an implementation strategy consisting of specific training, PPH trolleys or carry cases, engagement of local champions, audits, and feedback. All components of the E-MOTIVE intervention can be performed by midwives.

 For all latest news, follow The Daily Star's Google News channel.
For all latest news, follow The Daily Star's Google News channel. 



Comments