Ozone: Good and bad
Ozone is a relatively unstable form of molecular oxygen containing three oxygen atoms (O3) and its production occurs in two layers of the atmosphere, i.e. troposphere and stratosphere. It is blue in colour and has a strong odour. The production of ozone in ground level atmosphere (troposphere) is regarded negatively because it comes from pollutants, which result from industrial activities, transport operation and some natural sources. On the other hand, the natural production of ozone in the stratosphere is necessary because it plays a key role in protecting life on Earth from the ultraviolet rays of the sun. The major environmental problems we are facing now is its decreasing level in the stratosphere and the increasing level in the troposphere due to anthropogenic activities.
Stratospheric ozone
Stratosphere is a region of the atmosphere about 15 to 50 kilometres above the Earth's surface and about 90 percent of Earth's ozone production occurs in this layer. In the stratosphere the ozone layer extends from roughly 20 km to 48 km where the concentration of ozone range from about 2 to 8 parts per million. If all of the ozone were compressed to the pressure of air at sea level, it would be only a few millimeters thick. Ozone molecules are constantly created in chemical reactions caused when the ultraviolet radiation from the sunlight strikes the stratosphere.
At any given time, the average amount of ozone in the stratosphere remains fairly constant when the creative and destructive forces occur naturally. But this natural ozone has gradually been depleted by various human activities that release ozone-destroying chemicals into the atmosphere. The chemicals released into the atmosphere by industrial activities include chlorocarbon compounds (CCl4 and CH3Cl3), chlorofluorocarbon compounds or CFCs (CFCl3 and CF2Cl2) and halon compounds (CF3Br and CF2ClBr). Most of these chemical substances remain unchanged long enough to drift up to the stratosphere because they are chemically stable compounds containing halogen atoms, i.e. chlorine or bromine.
CFCs are used for a wide variety of industrial purposes, e.g. in refrigeration systems, air conditioners, aerosols, solvents and in the production of some types of packaging, because they are relatively inexpensive, highly effective, stable in the atmosphere and non-toxic to humans. But once CFCs reach the stratosphere, the solar ultraviolet radiation strikes them and release chlorine (Cl), which acts as a catalyst. The chlorine atom repeatedly combines with and breaks apart ozone molecules forming the single oxygen molecule and one chlorine monoxide molecule (ClO). Then, the chlorine monoxide molecule can combine with an oxygen atom to form an oxygen molecule and release the chlorine to begin the process all over again. Through this cycle, one chlorine atom can destroy up to 100,000 ozone molecules and deplete ozone much faster than nature can replace it.
Ozone effectively absorbs the Sun's harmful ultraviolet radiation(i.e. the most energetic ultraviolet light UV-C and UV-B). The protective role of the ozone layer is so vital that life on land probably would not have evolved - and could not exist today - without it.
As the stratospheric ozone layer is depleted, higher ultraviolet radiation reaches the earth's surface and harms human health, freshwater and marine ecosystems, reduces crop yields, and affects forests. The impacts of the increased UV levels include the increasing cases of skin cancers, cataracts, and impaired immune systems; decreasing growth and yields of some crops, such as corn, rice, cotton, beans, wheat, canola, barley, oats and soybeans; decreasing amount of single-celled plants, known as phytoplankton in the ocean, which could ultimately affect fish populations; reducing the construction materials used outdoor.
Tropospheric ozone
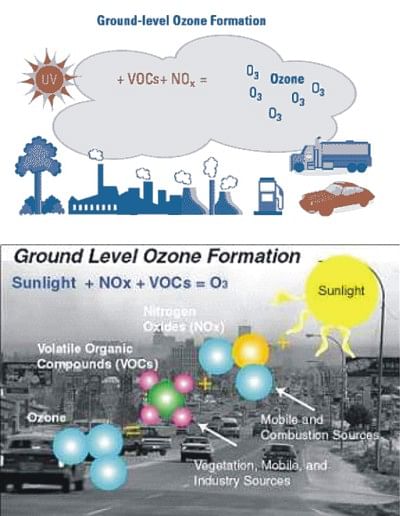
Troposphere is located 0-15 km above the Earth, it is known as the ground-level atmosphere layer. The ozone produced in this ground level is known as bad ozone. This ground-level ozone has two major sources, i.e. intrusion from the stratosphere and production from photochemical reactions. The tropospheric ozone plays several key roles in the atmosphere although it oxidises many chemical substances in troposphere. The tropospheric ozone is also a greenhouse gas (GHG) because it absorbs outgoing longwave radiation that contributes to the global warming.
Tropospheric ozone is formed by the interaction of sunlight, particularly ultraviolet light, with volatile organic compounds (VOCs) and nitrogen oxides (NOx), which are emitted by automobiles, gasoline vapours, fossil fuel power plants, refineries, and certain other industries. VOCs are organic compounds that contain carbon and hydrogen and can evaporate easily, e.g. octane, butane and sugar, which are mostly emitted by motor vehicles, vegetation, industry, commerce, dry cleaners, and paints. Nitrogen oxides like nitric oxide (NO) and nitrogen dioxide (NO2) are released into the atmosphere when fossil fuels like oil, coal and natural gas are burned. It is mostly emitted by motor vehicles, power plants, industrial facilities, biomass burning and lightning.
When released into the atmosphere, both VOCs and NOx can produce ozone and other harmful pollutants that lead to smog, which is sometimes called as photochemical smog or photochemical air pollution. One of the main components of photochemical smog is ozone.
The increased ground-level ozone causes harmful impacts on humans, plants, and materials. It may cause human health problems, e.g. eye irritation, breathing problems, lung damage, chest pain, headache or nausea, intensification of asthma symptoms, and increased cardio-respiratory deaths. Also, the ozone can harm plants, trees, and crops by preventing the plant from being able to use the sun's energy by reacting with the molecular links between the carbon atoms (called the carbon-carbon bonds) in the plant's photosynthetic mechanism. Furthermore, ground-level ozone affects materials by deteriorating and reducing the strength of products made of rubber and certain fabrics because ozone is a strong oxidant.
The problems of stratospheric ozone depletion and tropospheric ozone production are mostly due to anthropogenic activities that release manmade chemicals containing millions of tonnes of ozone depleting substances and tonnes of air pollutants. Although CFCs have been banned in many countries and replaced by HCFCs, which do deplete the ozone layer but not as quickly as CFCs.
In order to avoid those harmful effects, a series of international agreements to reduce the pace of ozone depletion in stratosphere and ozone production in troposphere have been held, e.g. the 1985 Vienna Convention on the Protection of the Ozone Layer and the 1987 Montreal Protocol on Substances that Deplete the Ozone Layer, which are arranged to freeze and decrease the production CFC to certain levels. This treaty entered into force on January 1, 1989. It is believed that if the international agreement is adhered to, the ozone layer is expected to recover by 2050.
But the scientific consensus of researchers is that it should immediately stop producing ozone-depleting chemicals. Even with immediate action, models indicate that it will take 50-60 years for the ozone layer to return to 1975 levels and another 100-200 years for full recovery to pre-1950 levels.
The problem of both stratospheric and tropospheric ozone is not easy to solve. Today, the scientists found that the most dangerous, among the many known "enemies" of the O3 layer, is N2O (nitrous oxide), constantly produced by human activities. Its "banning" will surely be more difficult.

 For all latest news, follow The Daily Star's Google News channel.
For all latest news, follow The Daily Star's Google News channel. 



Comments